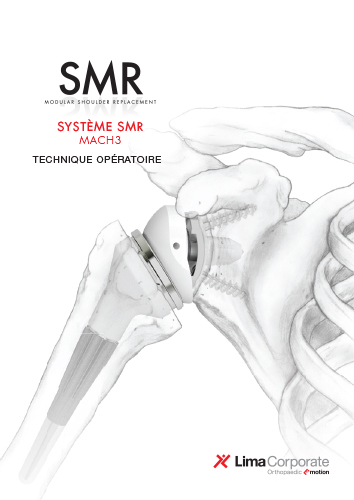
SMR Anatomic
Balanced by Design
Images

A propos
La prothèse d’épaule SMR Anatomique de LimaCorporate est conçue pour offrir une solution complète aux chirurgiens orthopédiques.
La modularité est une caractéristique clé de la plateforme SMR, qui permet de l’adapter à tous les types d’arthroplastie anatomique de l’épaule, des interventions programmées avec épargne osseuse aux interventions de traumatologie [2-8]. La plateforme SMR associée à une glène cimentée a obtenu la note ODEP 10A [1].
Voir la bibliographie
BIBLIOGRAPHIE
[1] 10A ODEP Rating for SMR Cemented Glenoid / Uncemented Humeral Stem. Latest ODEP Ratings can be found at www.odep.org.uk
[2] Kirsch JM, Khan M, Thornley P, Gichuru M, Freehill MT, Neviaser A, Moravek J, Miller BS, Bedi A. Platform shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2018;27(4):756-3.
[3] Weber-Spickschen TS, Alfke D, Agneskirchner JD. The use of a modular system to convert an anatomical total shoulder arthroplasty to a reverse shoulder arthroplasty: Clinical and radiological results. Bone Joint J. 2015 Dec;97-B(12):1662-7
[4] Castagna A, Delcogliano M, de Caro F, Ziveri G, Borroni M, Gumina S, Postacchini F, De Biase CF. Conversion of shoulder arthroplasty to reverse implants: clinical and radiological results using a modular system. Int Orthop. 2013 Jul;37(7):1297-305.
[5] Castagna A., Randelli M., Garofalo R., Maradei L., Giardella A., Borroni M. Mid-Term results of a metalbacked glenoid component in total shoulder replacement. J Bone Joint Surg [Br], 92(10): 1410-1415, 2010
[6] Ross M, Hope B, Stokes A, Peters SE, McLeod I, Duke PF. Reverse shoulder arthroplasty for the treatment of three-part and four-part proximal humeral fractures in the elderly. J Shoulder Elbow Surg. 2014 Aug 26
[7] Boyle MJ, Youn SM, Frampton CM, Ball CM. Functional outcomes of reverse shoulder arthroplasty compared with hemiarthroplasty for acute proximal humeral fractures. J Shoulder Elbow Surg. 2013 Jan;22(1):32-7.
[8] Martinez AA. Calvo A, Bejarano C, Carbonel I, Herrera A. The use of the Lima reverse shoulder arthroplasty for the treatment of fracture sequelae of the proximal humerus. J Orthop Sci. 2012 Mar;17(2):141–7.
[1] 10A ODEP Rating for SMR Cemented Glenoid / Uncemented Humeral Stem. Latest ODEP Ratings can be found at www.odep.org.uk
[2] Kirsch JM, Khan M, Thornley P, Gichuru M, Freehill MT, Neviaser A, Moravek J, Miller BS, Bedi A. Platform shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2018;27(4):756-3.
[3] Weber-Spickschen TS, Alfke D, Agneskirchner JD. The use of a modular system to convert an anatomical total shoulder arthroplasty to a reverse shoulder arthroplasty: Clinical and radiological results. Bone Joint J. 2015 Dec;97-B(12):1662-7
[4] Castagna A, Delcogliano M, de Caro F, Ziveri G, Borroni M, Gumina S, Postacchini F, De Biase CF. Conversion of shoulder arthroplasty to reverse implants: clinical and radiological results using a modular system. Int Orthop. 2013 Jul;37(7):1297-305.
[5] Castagna A., Randelli M., Garofalo R., Maradei L., Giardella A., Borroni M. Mid-Term results of a metalbacked glenoid component in total shoulder replacement. J Bone Joint Surg [Br], 92(10): 1410-1415, 2010
[6] Ross M, Hope B, Stokes A, Peters SE, McLeod I, Duke PF. Reverse shoulder arthroplasty for the treatment of three-part and four-part proximal humeral fractures in the elderly. J Shoulder Elbow Surg. 2014 Aug 26
[7] Boyle MJ, Youn SM, Frampton CM, Ball CM. Functional outcomes of reverse shoulder arthroplasty compared with hemiarthroplasty for acute proximal humeral fractures. J Shoulder Elbow Surg. 2013 Jan;22(1):32-7.
[8] Martinez AA. Calvo A, Bejarano C, Carbonel I, Herrera A. The use of the Lima reverse shoulder arthroplasty for the treatment of fracture sequelae of the proximal humerus. J Orthop Sci. 2012 Mar;17(2):141–7.
Tailles
& Options
Ressources
Clause De Non-Responsabilité
Veuillez noter que tous les produits ne sont pas disponibles et enregistrés sur tous les marchés. Veuillez contacter votre représentant commercial LimaCorporate pour toute information complémentaire.
BioBall® System est fabriqué par Merete GmbH et distribué par Lima France sas.
DiPHOS Nail est fabriqué par LSM-MED S.r.l. et distribué par Lima France sas.
BIOLOX® / BIOLOX®delta / BIOLOX OPTION® is a registered trademark of a company of the CeramTec Group, Germany.
BioBall® System est fabriqué par Merete GmbH et distribué par Lima France sas.
DiPHOS Nail est fabriqué par LSM-MED S.r.l. et distribué par Lima France sas.
BIOLOX® / BIOLOX®delta / BIOLOX OPTION® is a registered trademark of a company of the CeramTec Group, Germany.